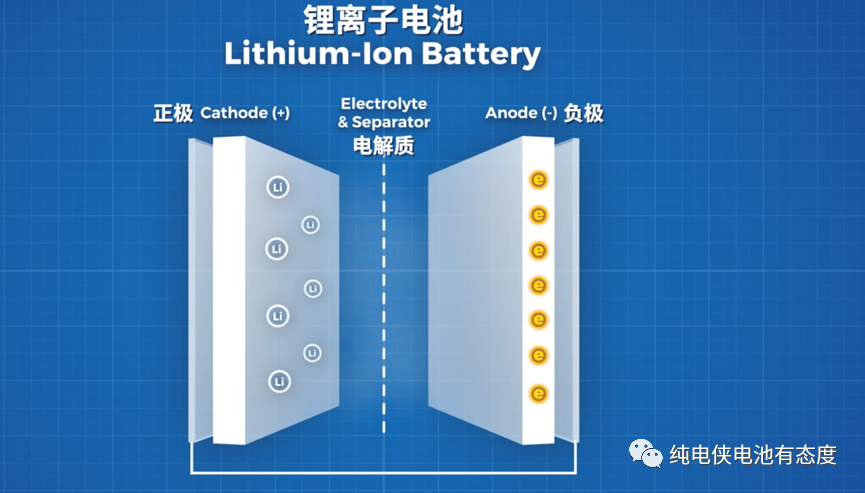
Both sodium ion and lithium ion are positively charged ions, in which the radius of sodium ion is 0.098 nm and that of lithium ion is 0.076 nm. Because the radius of sodium ion is much larger than that of lithium ion, it will lead to great structural changes in the process of sodium ion escaping and embedding in the negative electrode material, resulting in poor sodium storage performance and low reversible capacity of graphite negative electrode, which will seriously reduce the performance and life of sodium ion battery.
Although the graphite cathode material of lithium ion battery is not compatible with sodium ion battery, there are some similarities, and the charging and discharging principles are the same, so the cathode materials between them are similar. Compared with the graphite cathode of lithium ion battery, the cathode of sodium ion battery needs to meet the following conditions:
1, good energy storage and good reversibility; 2. The structural changes of sodium ions are small and the stability is good during the deintercalation process; 3. The potential after redox reaction is as close as possible to the potential of metallic sodium; 4, no side reaction with electrolyte, and good compatibility with electrolyte; 5. The raw materials are relatively easy to obtain, and mass commercial preparation can be carried out.
Hard carbon anode, which is also a carbon-based material, has become the first choice for sodium ion batteries because of its advantages.
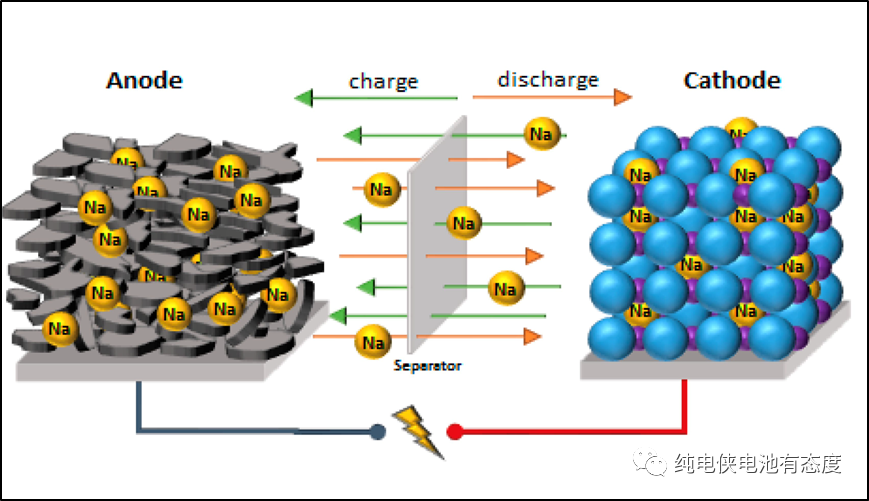
Hard carbon is the mainstream anode material of sodium ion battery, which is a carbon material produced by high temperature thermal decomposition, with larger pore structure and good conductivity. Compared with graphite cathode, hard carbon cathode has the following advantages:
1. The specific capacity is high. Compared with graphite negative electrode, hard carbon negative electrode can store more sodium ions per unit mass of material, which makes hard carbon negative electrode store more charges in the same volume, thus improving the energy density and endurance of the battery.
2. Excellent cycle stability. Because the pore structure of hard carbon is larger, it can contain more sodium ions, and the expansion and contraction of the electrode are more uniform during discharge, which increases the cycle stability of the hard carbon negative electrode and increases the life of the sodium ion battery.
However, even though the material has many advantages, the cost is always a hurdle in large-scale commercial production, and the main reason that restricts the development of sodium ion hard carbon anode is the cost.
According to the calculation, the cost of hard carbon negative electrode accounts for 16% of the total cost of raw materials for sodium ion batteries, while the cost of graphite negative electrode is only 11% of the total cost of raw materials for lithium batteries. The cost of hard carbon negative electrode is much higher than that of graphite negative electrode. Therefore, reducing the cost of hard carbon anode is an important goal of commercialization of sodium battery.